![Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex. Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex.](https://haygot.s3.amazonaws.com/questions/1048239_879440_ans_9590dd8f7eff475e873fdfa26f547f7a.PNG)
Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex.
Draw the structures of [Co(NH3)6]^3+, [Ni(CN)4]^2- and [Ni(CO)4]. - Sarthaks eConnect | Largest Online Education Community
![Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(2+) is an outer orbital complex ? Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(2+) is an outer orbital complex ?](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)
Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(2+) is an outer orbital complex ?
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1090/0*rkWRh7JsXBvtOfdK.jpg)
Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory. Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.](https://haygot.s3.amazonaws.com/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.
![Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:598/0*gC7TqoG4Szy6QwnB.jpg)
Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4] Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4]](https://i.ytimg.com/vi/VpeKIvrO0ZQ/maxresdefault.jpg)
Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4]
![निम्नलिखित के I.U.P.A.C. नाम लिखिए - (i) `[Co(NH_(3))_(5)]Cl_(2)` (ii) `[Pt(NH_(3))_(2)Cl(No2)]` - YouTube निम्नलिखित के I.U.P.A.C. नाम लिखिए - (i) `[Co(NH_(3))_(5)]Cl_(2)` (ii) `[Pt(NH_(3))_(2)Cl(No2)]` - YouTube](https://i.ytimg.com/vi/MEn7BzyQgW8/maxresdefault.jpg)
निम्नलिखित के I.U.P.A.C. नाम लिखिए - (i) `[Co(NH_(3))_(5)]Cl_(2)` (ii) `[Pt(NH_(3))_(2)Cl(No2)]` - YouTube
✓ Solved: A solution is prepared by adding 0.10 mole of Ni(NH3)6 Cl2 to 0.50 L of 3.0 M NH3 . Calculate...

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
![Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4] Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/VnBlS0l2ck8wWlE=/sd/)
Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4]
![Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] from Chemistry Coordination Compounds Class 12 Nagaland Board Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] from Chemistry Coordination Compounds Class 12 Nagaland Board](https://www.zigya.com/application/zrc/images/qvar/CHEN12070315.png)
![Síntese e caracterização do [Ni (NH3) 6]Cl2 - relatório de inorganica experimental. | Docsity Síntese e caracterização do [Ni (NH3) 6]Cl2 - relatório de inorganica experimental. | Docsity](https://static.docsity.com/documents_first_pages/notas/2011/08/29/980338e448e2c05e3d923b4e8cdb1a7a.png)
![Дать расчет степени окисления [Ni(NH3)6]Cl2 - Школьные Знания.com Дать расчет степени окисления [Ni(NH3)6]Cl2 - Школьные Знания.com](https://ru-static.z-dn.net/files/d35/3ff3c3f51a2665c4d88fbe38629de25d.jpg)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-f7fd36508df0305723aa4956637d3097.webp)
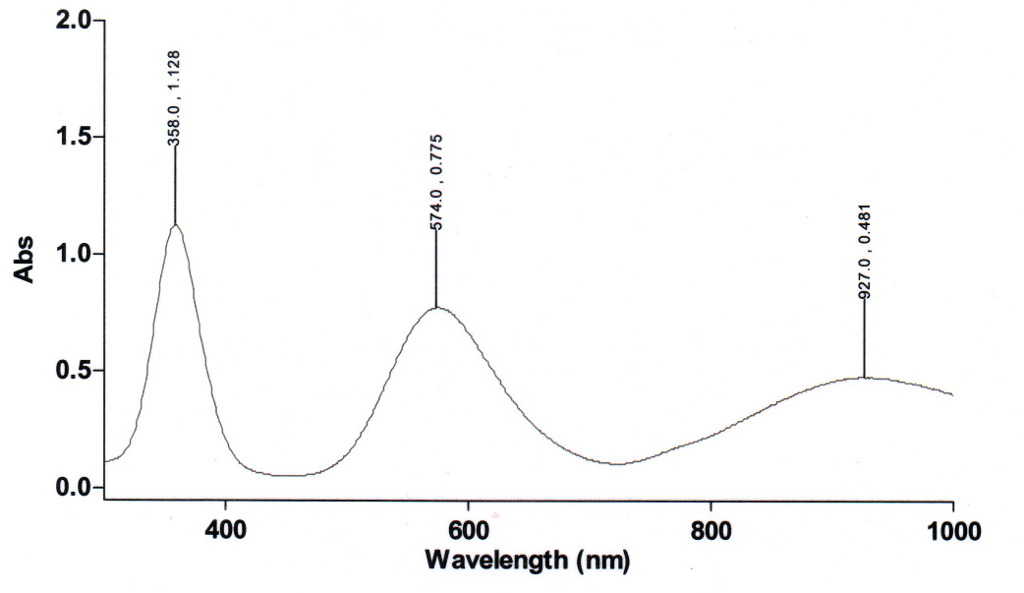
![The magnetic moment of the complex [Ni(NH3)6] Cl2 is: - The magnetic moment of the complex [Ni(NH3)6] Cl2 is: -](https://dwes9vv9u0550.cloudfront.net/images/1532429/12cd2591-0143-4485-9682-c054f62bb397.jpg)
![PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2 PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2](https://i1.rgstatic.net/publication/256461217_Synthesis_structure_and_thermal_decomposition_of_NiNH3_6VOO22NH32/links/598c15b1458515c333a5e1ff/largepreview.png)
![Practice Work Sheet :-1 [Chemistry –XII {CBSE}]: -CH Practice Work Sheet :-1 [Chemistry –XII {CBSE}]: -CH](https://s3.studylib.net/store/data/008635007_1-de6c1b8d5d5e0bacf5c03f5e5d601818.png)

![What is the IUPAC name of the complex [Ni(NH3)6]Cl2 | Coordination Chemistry Questions - YouTube What is the IUPAC name of the complex [Ni(NH3)6]Cl2 | Coordination Chemistry Questions - YouTube](https://i.ytimg.com/vi/GtBNjFWTE88/maxresdefault.jpg)
![How is Cr(NH3) 6] 3+ paramagnetic and [Co(NH3) 6] 3+ is diamagnetic? - Quora How is Cr(NH3) 6] 3+ paramagnetic and [Co(NH3) 6] 3+ is diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-5256b41f5879418f6611bf7cb42000b7.webp)

2 with gaseous NH3; crystal growth via in-situ solvation PDF) Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation](https://i1.rgstatic.net/publication/269400063_Reaction_of_NiH2O6NO32_with_gaseous_NH3_crystal_growth_via_in-situ_solvation/links/59d286db0f7e9b4fd7fc8f02/largepreview.png)